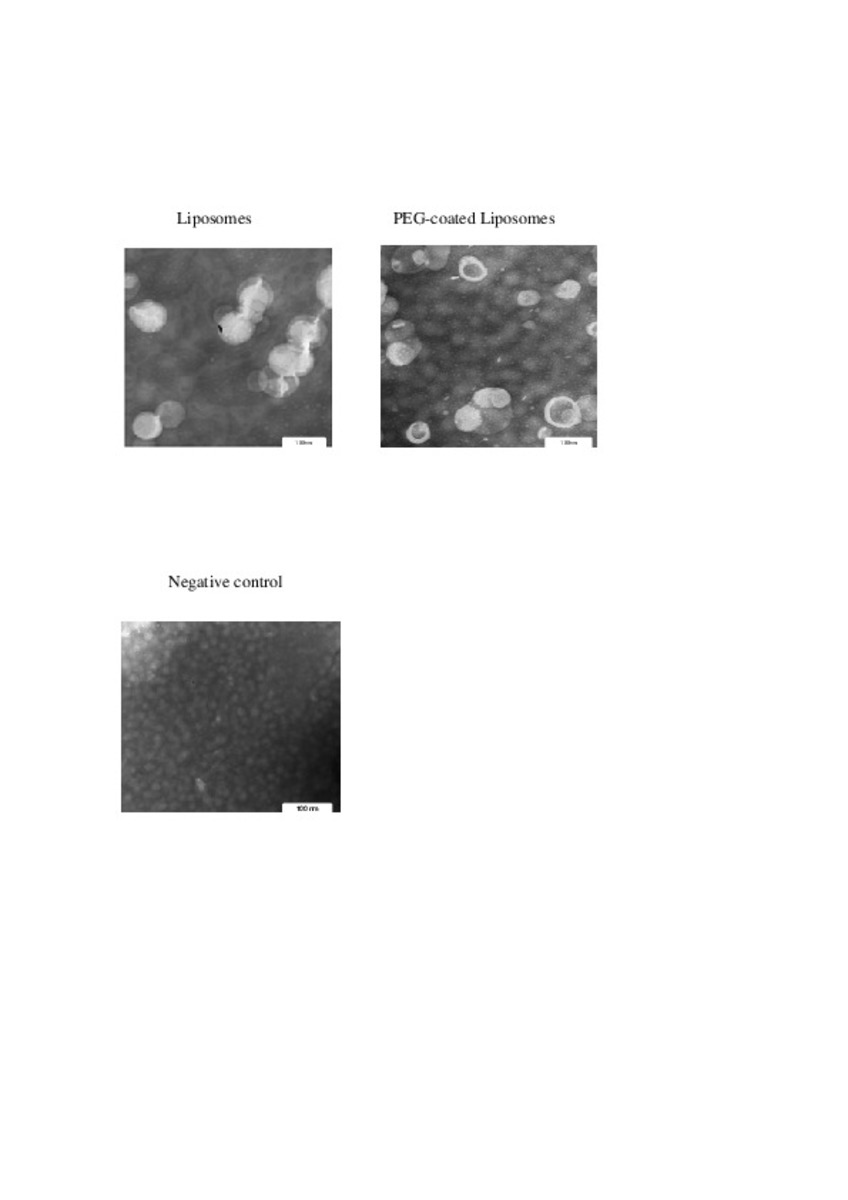
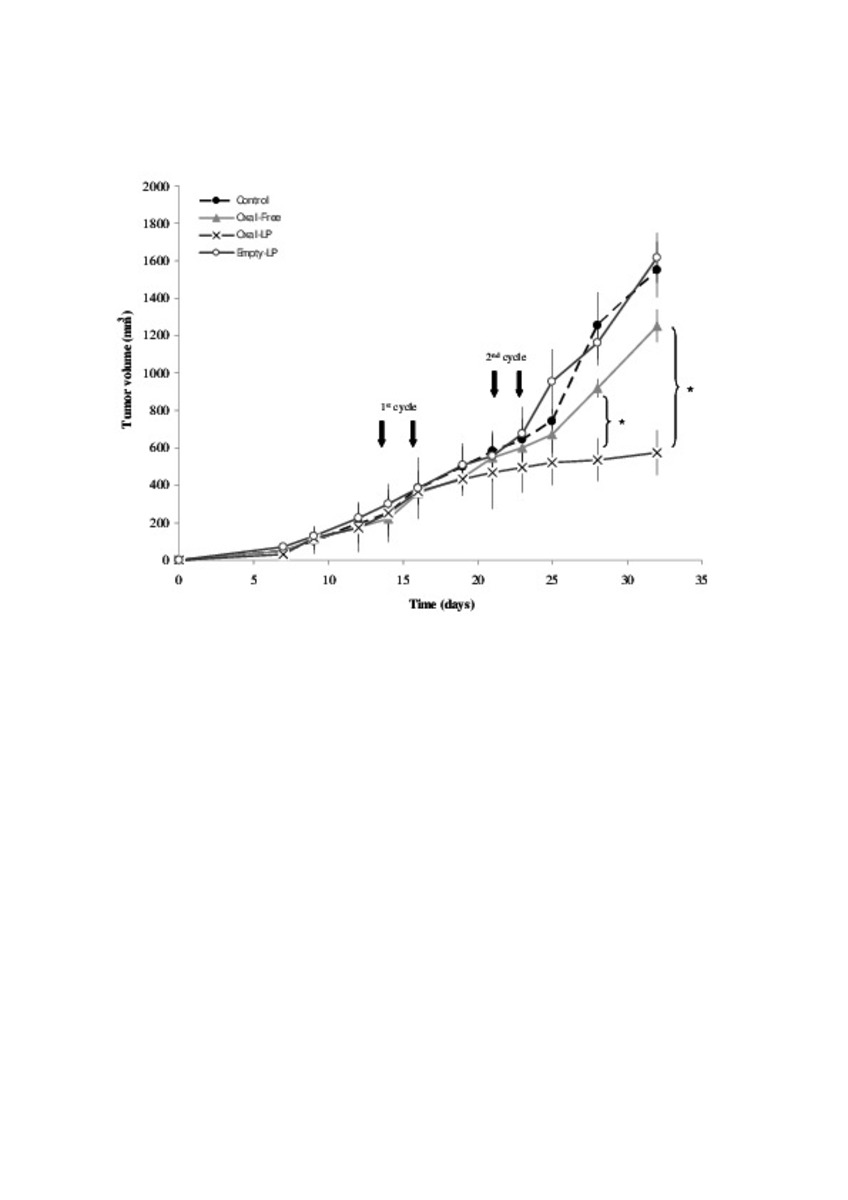
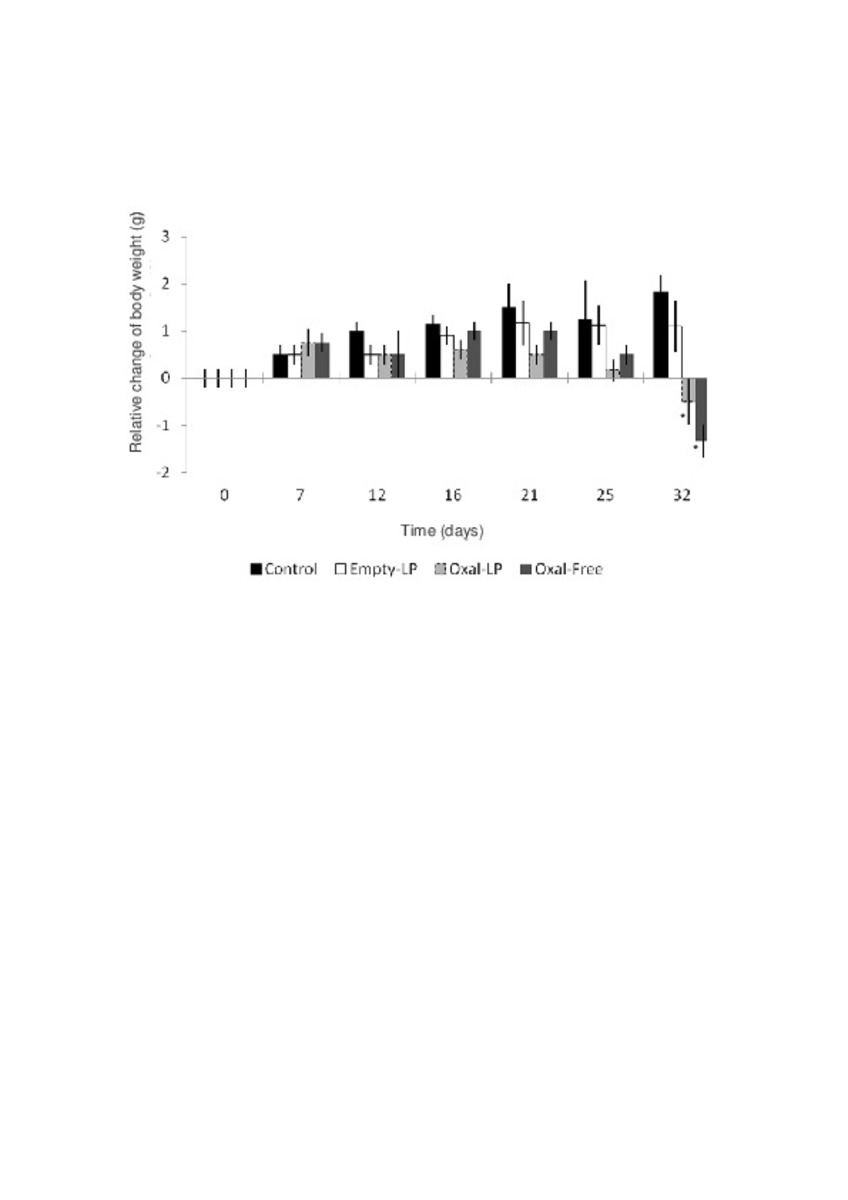
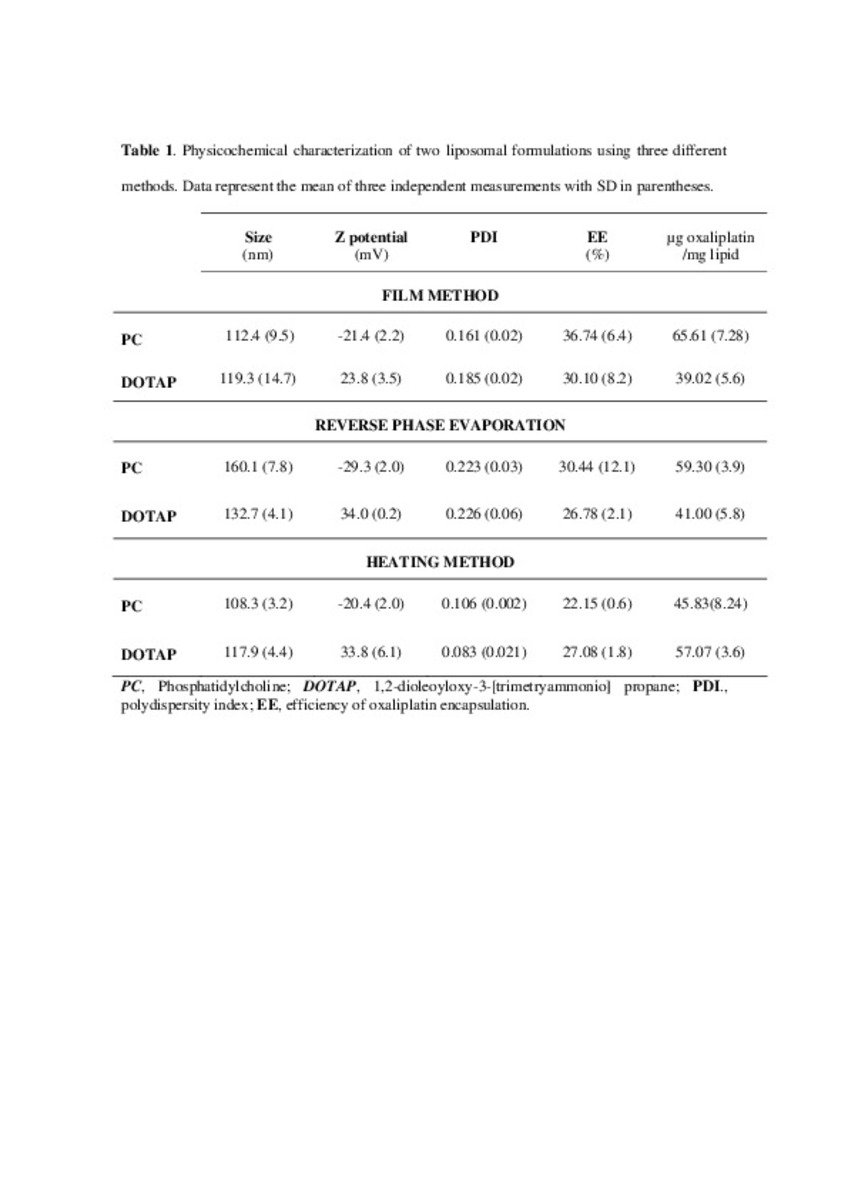
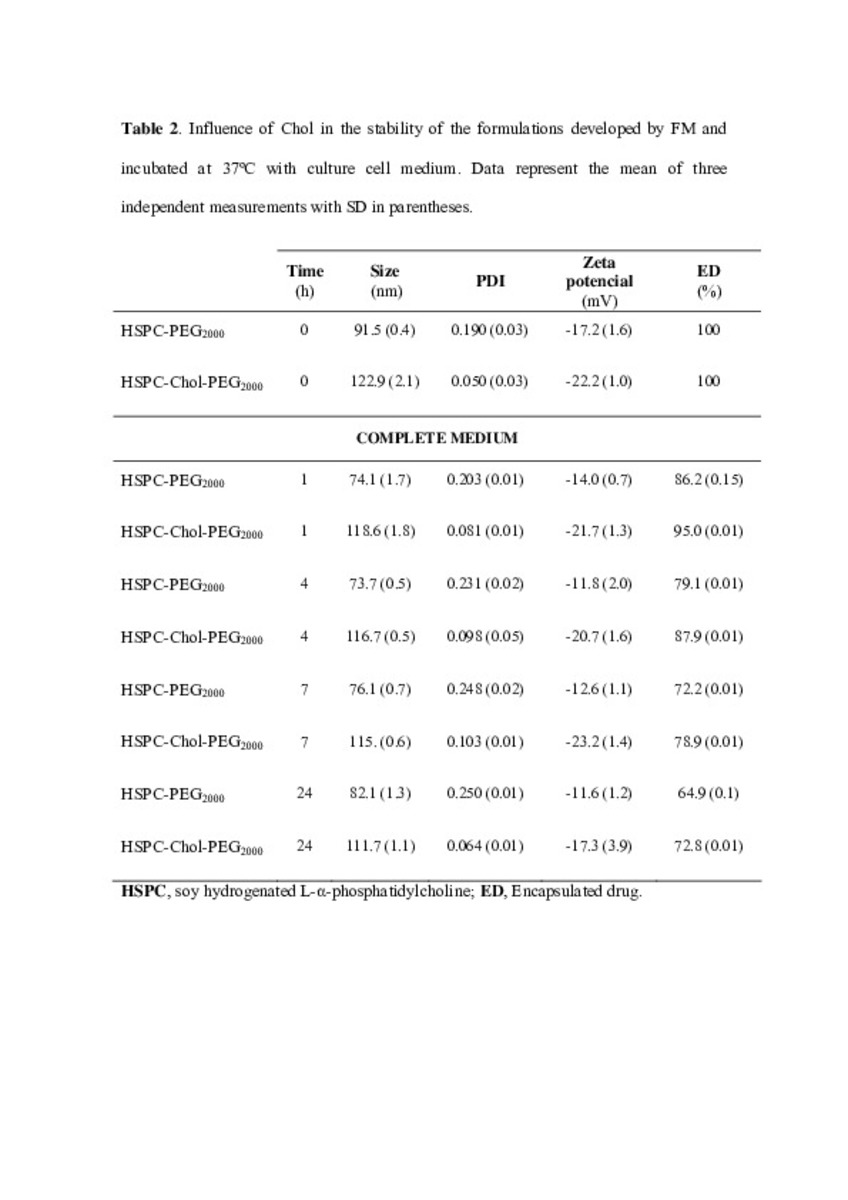
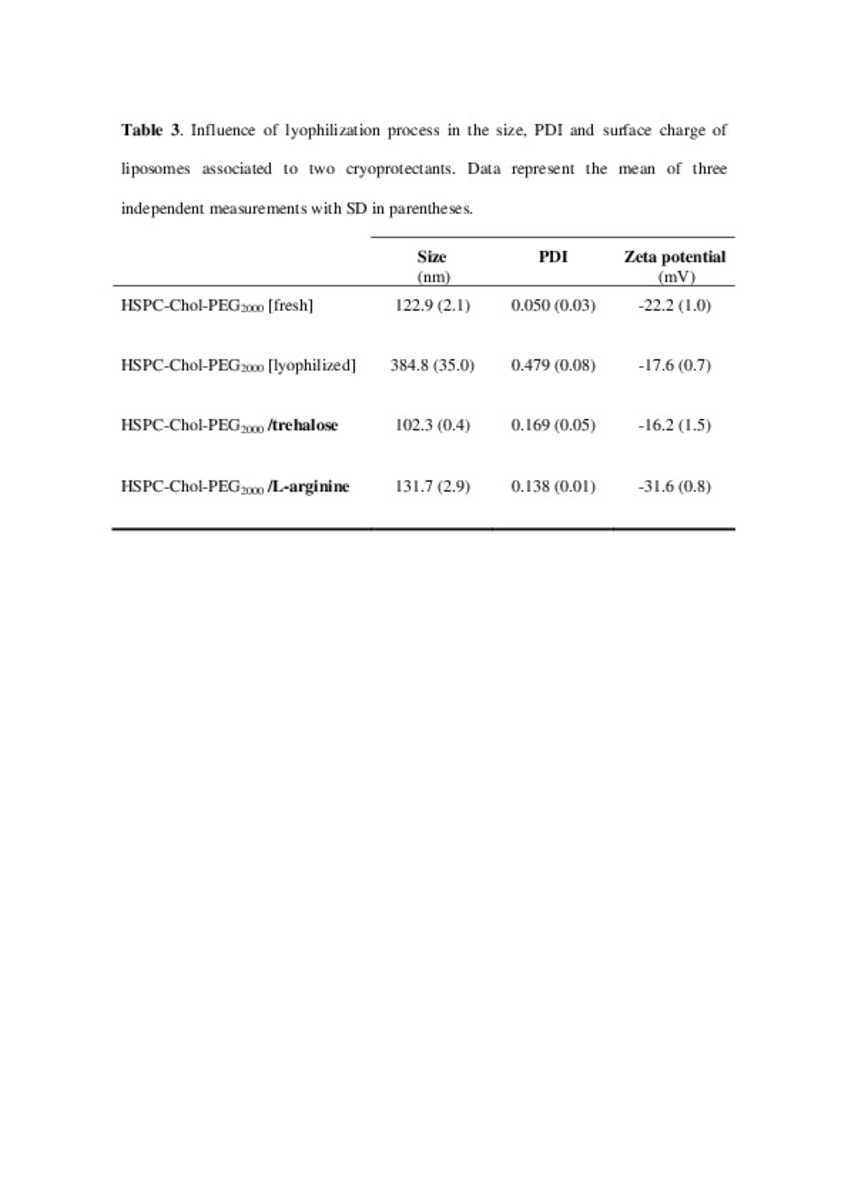
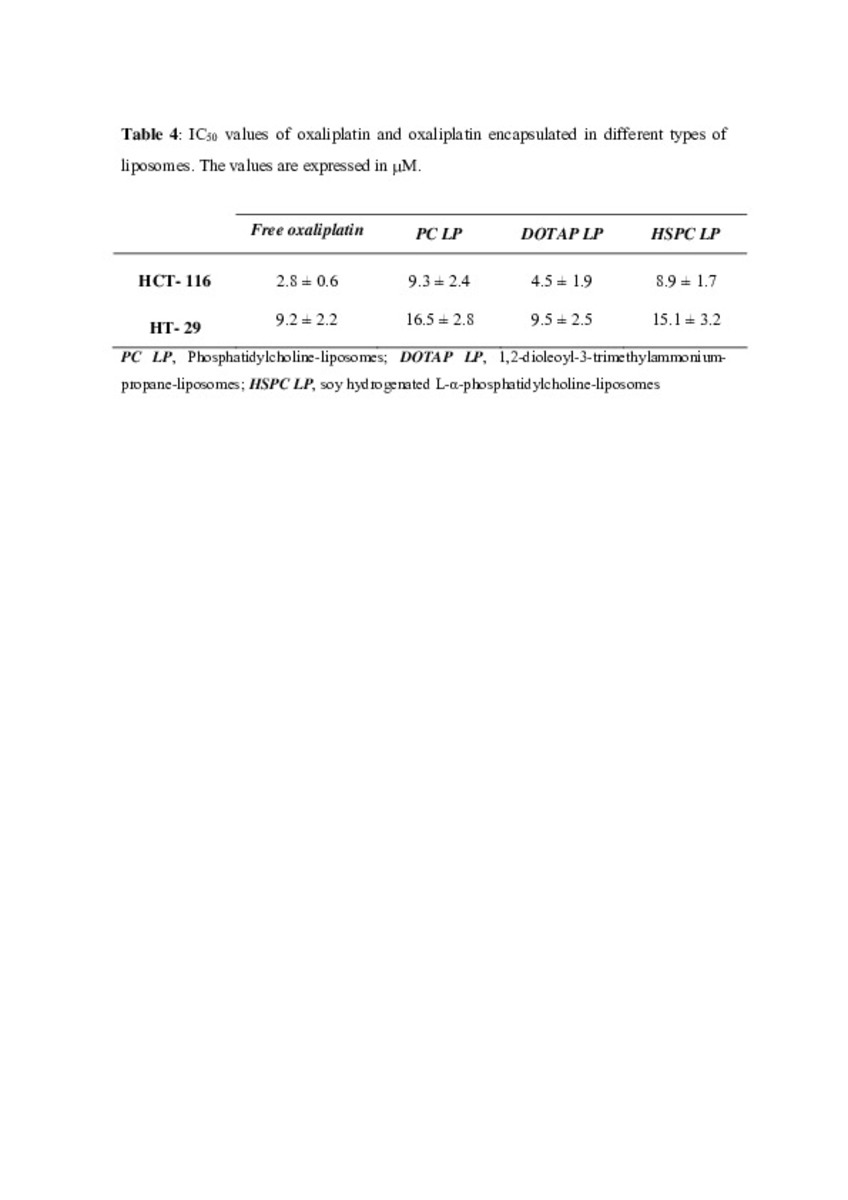
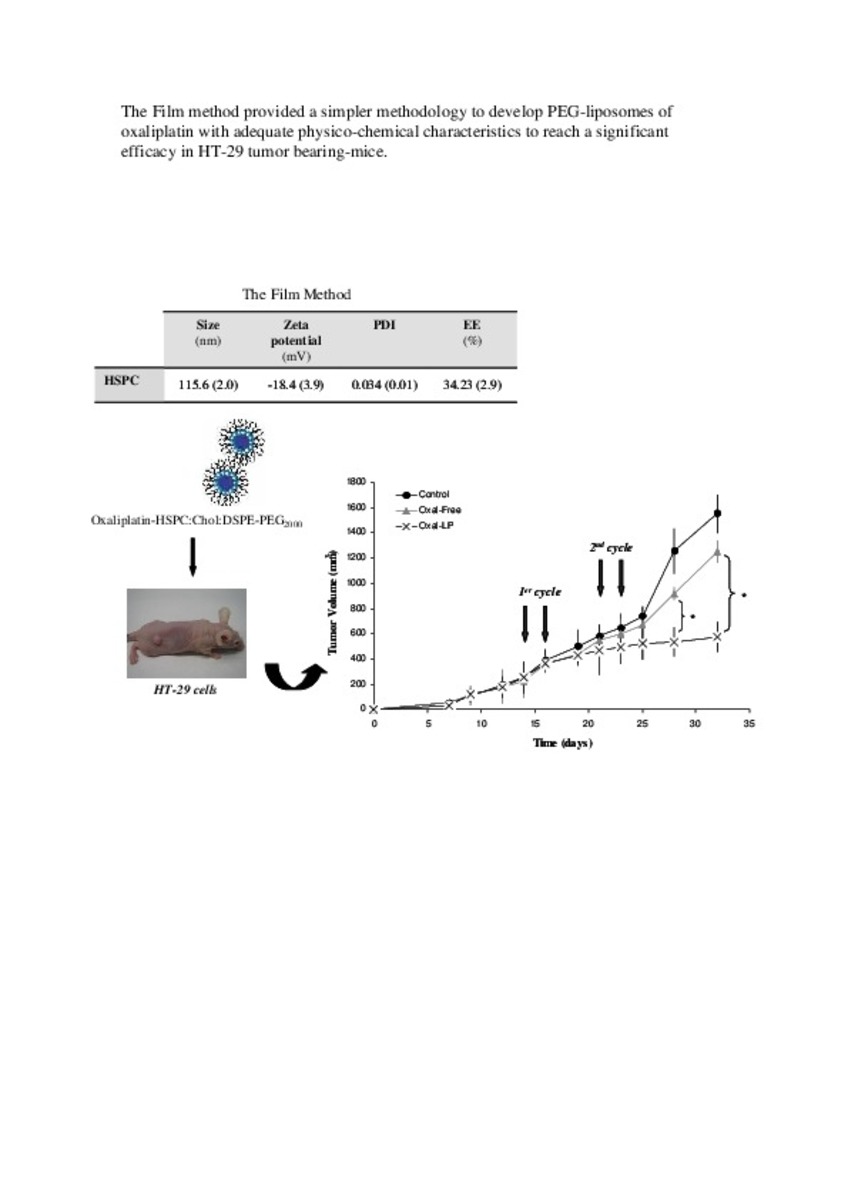
Application of different methods to formulate PEG-liposomes of oxaliplatin: Evaluation in vitro and in vivo
Keywords:
Film method
Reverse-Phase evaporation method
Heating method
PEG-coated liposomes
Oxaliplatin
Citation:
Zalba S, Navarro I, Troconiz IF, Ilarduya CT, Garrido MJ. Application of different methods to formulate PEG-liposomes of oxaliplatin: Evaluation in vitro and in vivo. Eur J Pharm Biopharm. 2012 Feb 18.
Statistics and impact
0 citas en

0 citas en

Items in Dadun are protected by copyright, with all rights reserved, unless otherwise indicated.