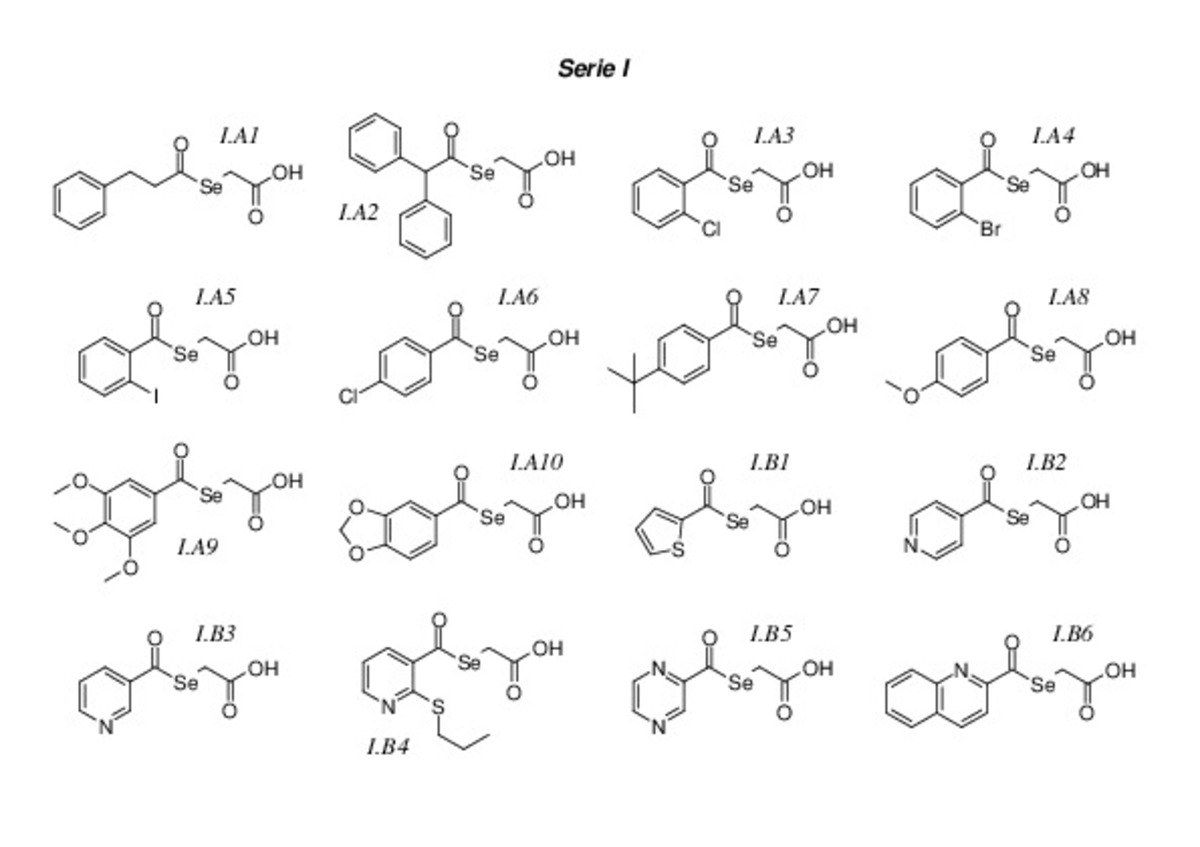
Diseño, síntesis y evaluación biológica de nuevos selenoésteres con actividad antiproliferativa, citotóxica y quimiopreventiva
Keywords:
Materias Investigacion::Química::Química orgánica
Materias Investigacion::Farmacia::Química farmacéutica
Estudio de nuevos fármacos
Diseño síntesis
Organometalicos
Defense Date:
14-Dec-2012
Publisher:
Servicio de Publicaciones de la Universidad de Navarra
Citation:
DOMINGUEZ ALVAREZ, E. “Diseño, síntesis y evaluación biológica de nuevos selenoésteres con actividad antiproliferativa, citotóxica y quimiopreventiva”. Palop-Cubillo, J.A. y Sanmartín-Grijalba, C. (dirs.). Tesis doctoral. Universidad de Navarra, Pamplona, 2014
Statistics and impact
0 citas en

0 citas en

Items in Dadun are protected by copyright, with all rights reserved, unless otherwise indicated.