Full metadata record
| DC Field | Value | Language |
|---|---|---|
| dc.creator | Kudo, M. (Masatoshi) | - |
| dc.creator | Matilla, A. (Ana) | - |
| dc.creator | Santoro, A. (Armando) | - |
| dc.creator | Melero, I. (Ignacio) | - |
| dc.creator | Cubillo-Gracián, A. (Antonio) | - |
| dc.creator | Acosta-Rivera, M. (Mirelis) | - |
| dc.creator | Choo, S.P. (Su-Pin) | - |
| dc.creator | El-Khoueiry, A. (Anthony) | - |
| dc.creator | Kuromatsu, R. (Ryoko) | - |
| dc.creator | El-Rayes, B.F. (Bassel F.) | - |
| dc.creator | Numata, K. (Kazushi) | - |
| dc.creator | Itoh, Y. (Yoshito) | - |
| dc.creator | Di-Costanzo, F. (Francesco) | - |
| dc.creator | Crysler, O. (Oxana) | - |
| dc.creator | Reig, M. (Maria) | - |
| dc.creator | Shen, Y. (Yun) | - |
| dc.creator | Neely, J. (Jaclyn) | - |
| dc.creator | Tschaika, M. (Marina) | - |
| dc.creator | Wisniewski, T. (Tami) | - |
| dc.creator | Sangro, B. (Bruno) | - |
| dc.date.accessioned | 2024-02-02T12:37:53Z | - |
| dc.date.available | 2024-02-02T12:37:53Z | - |
| dc.date.issued | 2021 | - |
| dc.identifier.citation | Kudo, M. (Masatoshi); Matilla, A. (Ana); Santoro, A. (Armando); et al. "CheckMate 040 cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis". Journal of Hepatology. 75 (3), 2021, 600 - 609 | es |
| dc.identifier.issn | 1600-0641 | - |
| dc.identifier.uri | https://hdl.handle.net/10171/68723 | - |
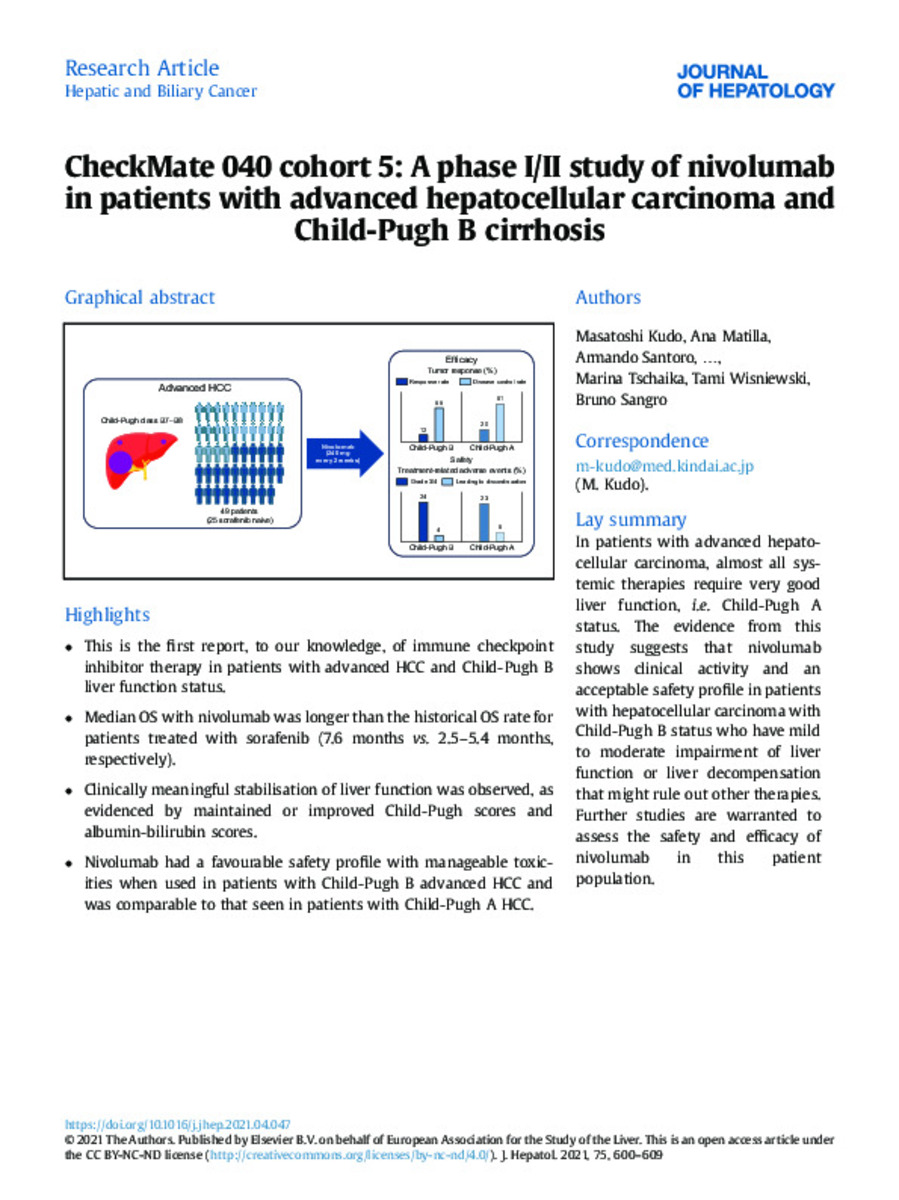
| dc.description.abstract | Background & Aims: Patients with advanced hepatocellular carcinoma (aHCC) and Child-Pugh B liver function are often excluded from clinical trials. In previous studies, overall survival for these patients treated with sorafenib was 3–5 months; thus, new treatments are needed. Nivolumab, alone or in combination with ipilimumab, is conditionally approved in the United States to treat patients with aHCC who previously received sorafenib. We describe nivolumab monotherapy outcomes in patients with Child-Pugh B status. Methods: This phase I/II, open-label, non-comparative, multicentre trial (27 centres) included patients with Child-Pugh B (B7–B8) aHCC. Patients received intravenous nivolumab 240 mg every 2 weeks until unacceptable toxicity or disease progression. Primary endpoints were objective response rate (ORR) by investigator assessment (using Response Evaluation Criteria in Solid Tumors v1.1) and duration of response. Safety was assessed using National Cancer Institute Common Terminology Criteria for Adverse Events v4.0. Results: Twenty-five sorafenib-naive and 24 sorafenib-treated patients began treatment between November 2016 and October 2017 (median follow-up, 16.3 months). Investigatorassessed ORR was 12% (95% CI 5–25%) with 6 patients responding; disease control rate was 55% (95% CI 40–69%). Median time to response was 2.7 months (interquartile range, 1.4–4.2), and median duration of response was 9.9 months (95% CI 9.7–9.9). Treatment-related adverse events (TRAEs) were reported in 25 patients (51%) and led to discontinuation in 2 patients (4%). The most frequent grade 3/4 TRAEs were hypertransaminasemia (n = 2), amylase increase (n = 2), and aspartate aminotransferase increase (n = 2). The safety of nivolumab was comparable to that in patients with Child-Pugh A aHCC. Conclusions: Nivolumab showed clinical activity and favourable safety with manageable toxicities, suggesting it could be suitable for patients with Child-Pugh B aHCC. Lay summary: In patients with advanced hepatocellular carcinoma, almost all systemic therapies require very good liver function, i.e. Child-Pugh A status. The evidence from this study suggests that nivolumab shows clinical activity and an acceptable safety profile in patients with hepatocellular carcinoma with Child-Pugh B status who have mild to moderate impairment of liver function or liver decompensation that might rule out other therapies. Further studies are warranted to assess the safety and efficacy of nivolumab in this patient population. | es_ES |
| dc.description.sponsorship | This study was supported by Bristol Myers Squibb (Princeton, NJ) and by Ono Pharmaceutical Co., Ltd. (Osaka, Japan). | es_ES |
| dc.language.iso | eng | es_ES |
| dc.publisher | Elsevier BV | es_ES |
| dc.rights | info:eu-repo/semantics/openAccess | es_ES |
| dc.subject | Liver cancer | es_ES |
| dc.subject | Objective response | es_ES |
| dc.subject | Overall survival | es_ES |
| dc.subject | Checkpoint inhibitors | es_ES |
| dc.subject | Anti-PD-1 | es_ES |
| dc.subject | Immunotherapy | es_ES |
| dc.subject | Liver decompensation | es_ES |
| dc.title | CheckMate 040 cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis | es_ES |
| dc.type | info:eu-repo/semantics/article | es_ES |
| dc.description.note | This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). | es_ES |
| dc.identifier.doi | 10.1016/j.jhep.2021.04.047 | - |
| dadun.citation.endingPage | 609 | es_ES |
| dadun.citation.number | 3 | es_ES |
| dadun.citation.publicationName | Journal of Hepatology | es_ES |
| dadun.citation.startingPage | 600 | es_ES |
| dadun.citation.volume | 75 | es_ES |
| dc.identifier.pmid | 34051329 | - |
Files in This Item:
Statistics and impact
Items in Dadun are protected by copyright, with all rights reserved, unless otherwise indicated.