Full metadata record
| DC Field | Value | Language |
|---|---|---|
| dc.creator | Estella-Hermoso-de-Mendoza, A. (Ander) | |
| dc.creator | Rayo, M. (Marta) | |
| dc.creator | Mollinedo, F. (Faustino) | |
| dc.creator | Blanco-Prieto, M.J. (María José) | |
| dc.date.accessioned | 2011-11-15T19:33:05Z | - |
| dc.date.available | 2011-11-15T19:33:05Z | - |
| dc.date.issued | 2008-02 | - |
| dc.identifier.citation | Estella-Hermoso de Mendoza A, Rayo M, Mollinedo F, Blanco-Prieto MJ. Lipid nanoparticles for alkyl lysophospholipid edelfosine encapsulation: development and in vitro characterization. Eur J Pharm Biopharm 2008 Feb;68(2):207-213. | es_ES |
| dc.identifier.issn | 0939-6411 | - |
| dc.identifier.uri | https://hdl.handle.net/10171/19830 | - |
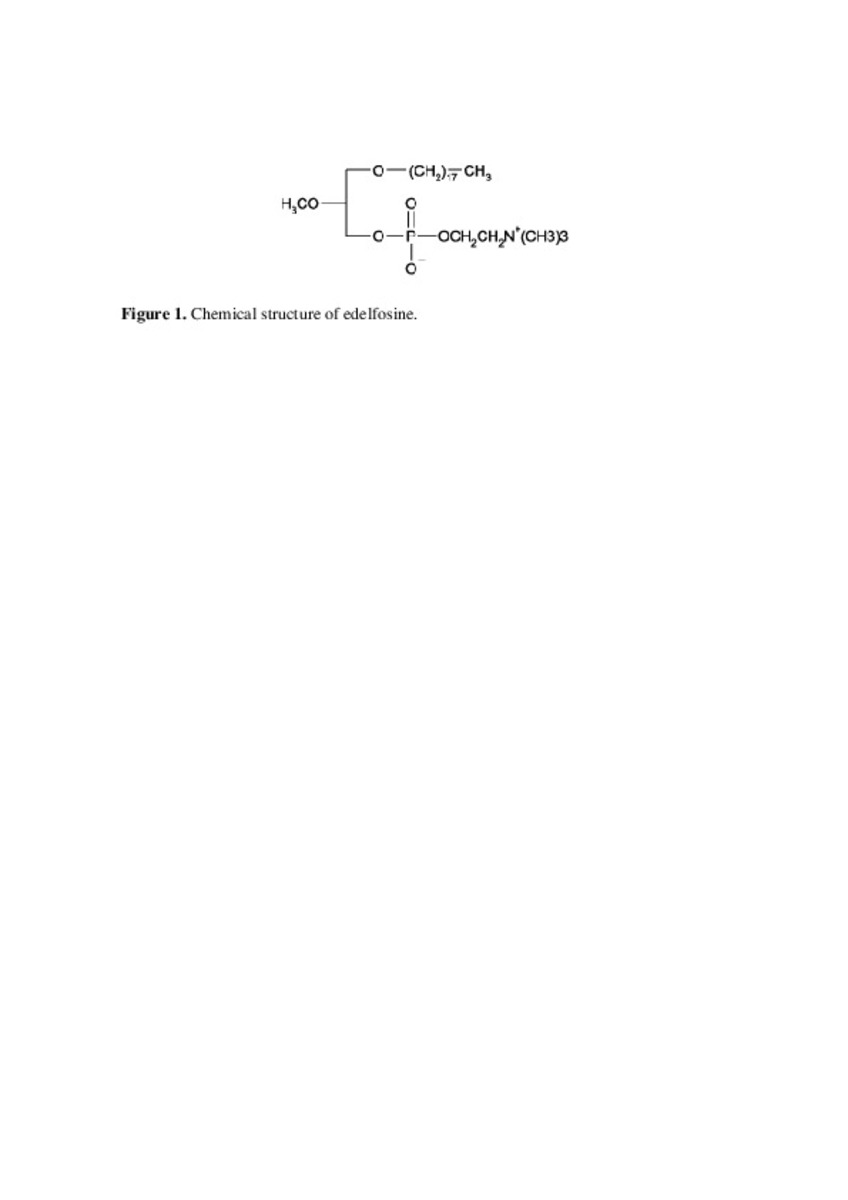
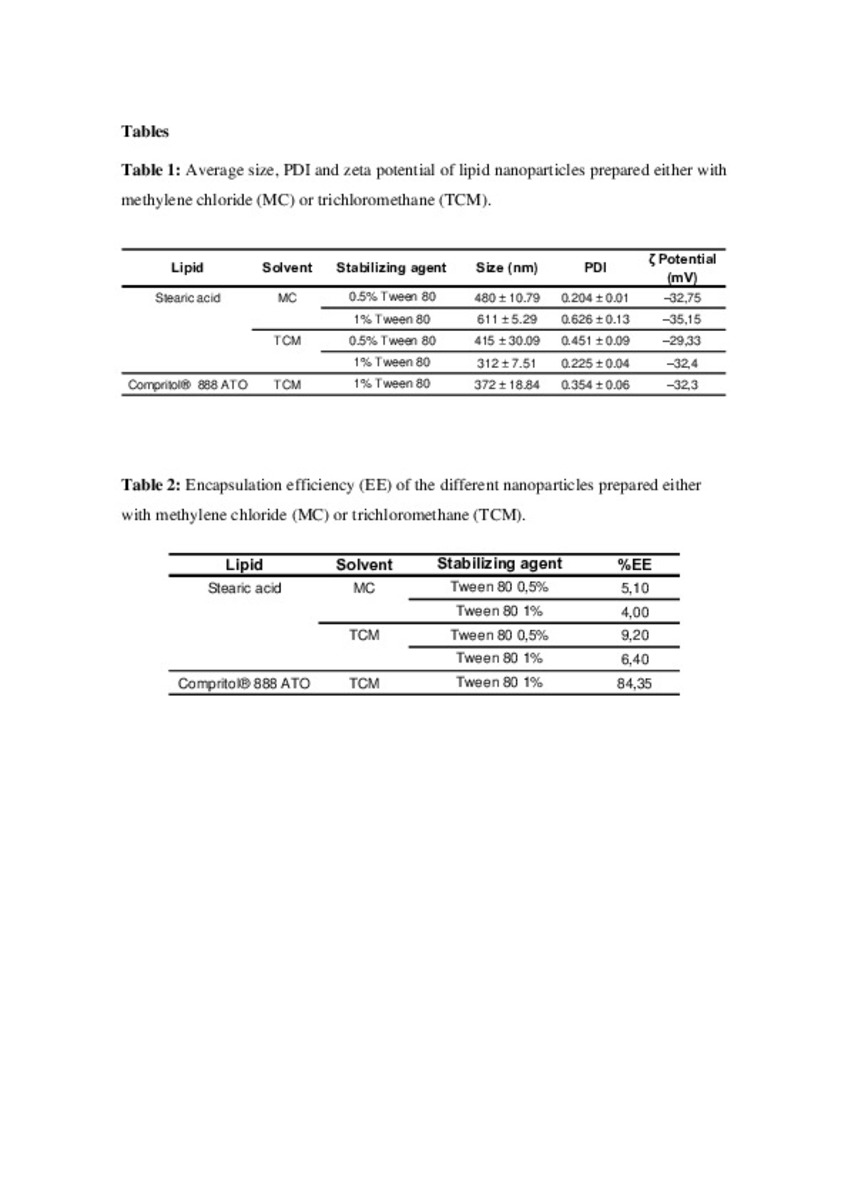
| dc.description.abstract | The ether lipid 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine, edelfosine (ET-18-OCH3) is the prototype molecule of a promising class of antitumour drugs named alkyl–lysophospholipid analogues (ALPs) or antitumor ether lipids. This drug presents a very important drawback as can be the dose depending haemolysis when administered intravenously. Lipid nanoparticles have been lately proposed for different drug encapsulation as an alternative to other controlled release delivery systems, such as liposomes or polymeric nanoparticles. The aim of this study was to develop a lipid nanoparticulate system that would decrease systemic toxicity as well as improve the therapeutic potential of the drug. Lipids employed were Compritol® 888 ATO and stearic acid. The nanoparticles were characterized by photon correlation spectroscopy for size and size distribution, and atomic force microscopy (AFM) was used for the determination of morphological properties. By both differential scanning calorimetry (DSC) and X-ray diffractometry, crystalline behaviour of lipids and drug was assessed. The drug encapsulation efficiency and the drug release kinetics under in vitro conditions were measured by HPLC–MS. It was concluded that Compritol® presents advantages as a matrix material for the manufacture of the nanoparticles and for the controlled release of edelfosine. | es_ES |
| dc.language.iso | eng | es_ES |
| dc.publisher | Elsevier | es_ES |
| dc.rights | info:eu-repo/semantics/openAccess | es_ES |
| dc.subject | Lipid nanoparticles | es_ES |
| dc.subject | Edelfosine | es_ES |
| dc.subject | Drug delivery | es_ES |
| dc.subject | Atomic force microscopy | es_ES |
| dc.subject | Differential scanning calorimetry | es_ES |
| dc.subject | X–ray diffractometry | es_ES |
| dc.title | Lipid nanoparticles for alkyl lysophospholipid edelfosine encapsulation: development and in vitro characterization | es_ES |
| dc.type | info:eu-repo/semantics/article | es_ES |
| dc.identifier.doi | http://dx.doi.org/10.1016/j.ejpb.2007.06.015 | es_ES |
Statistics and impact
Items in Dadun are protected by copyright, with all rights reserved, unless otherwise indicated.