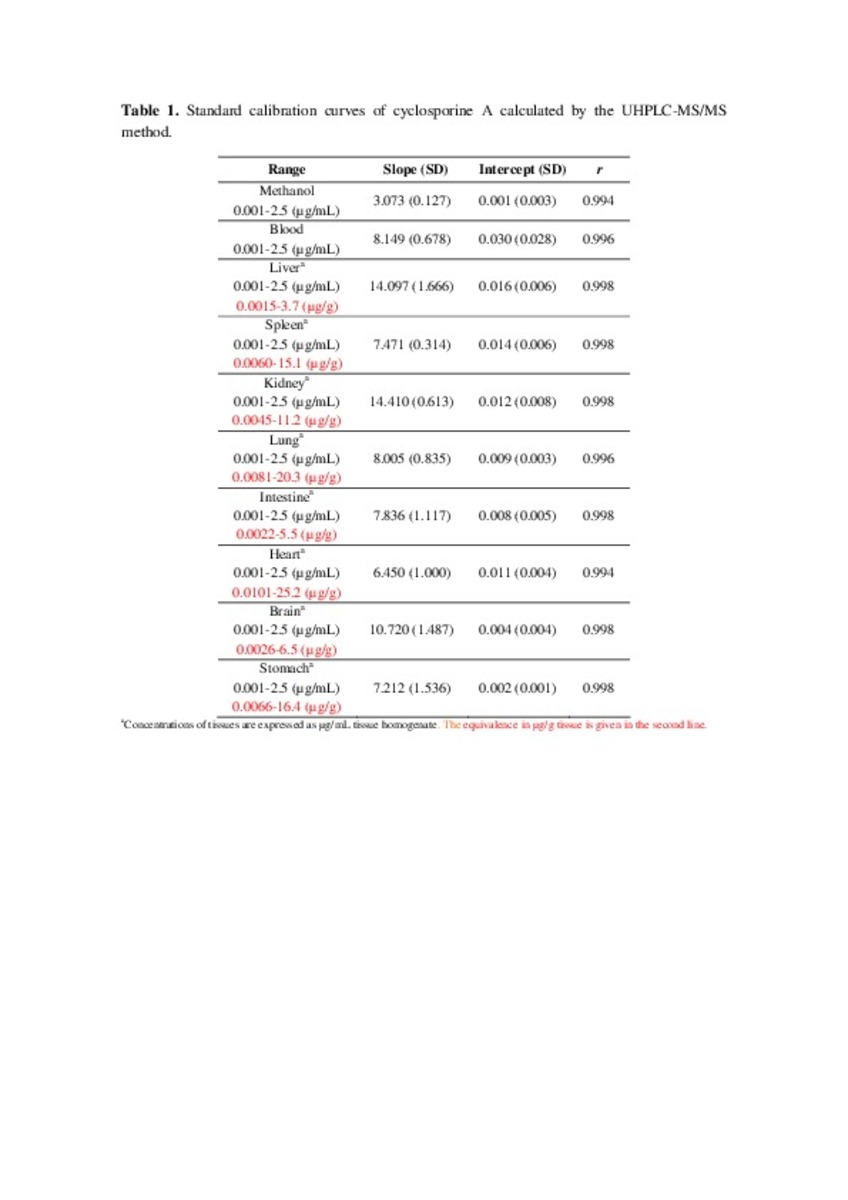
Ultra high performance liquid chromatography–tandem mass spectrometry method for cyclosporine a quantification in biological samples and lipid nanosystems
Palabras clave :
Biodistribution
Pharmacokinetics
Lipid nanocarriers
UHPLC–tandem mass spectrometry
Cyclosporine A
Fecha de publicación :
2013
Cita:
Guada M, Imbuluzqueta E, Estella-Hermoso de Mendoza A, Lana H, Dios-Viéitez MC, Blanco-Prieto MJ. Ultra high performance liquid chromatography–tandem mass spectrometry method for cyclosporine a quantification in biological samples and lipid nanosystems. J Chromatogr B Analyt Technol Biomed Life Sci 2013 5/15;927(0):164-172
Aparece en las colecciones:
Estadísticas e impacto
0 citas en

Los ítems de Dadun están protegidos por copyright, con todos los derechos reservados, a menos que se indique lo contrario.